Infectious Diseases
Accessible Respiratory
Virus Diagnostics
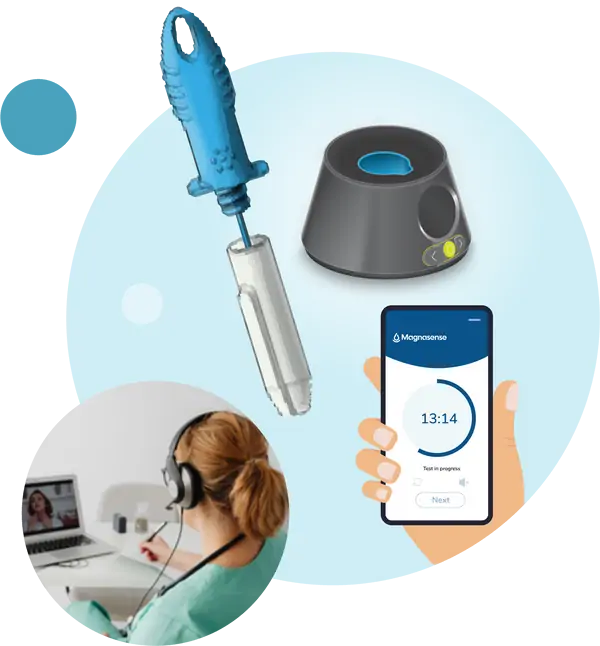
The landscape of self-administered diagnostics has evolved significantly, especially in the wake of the COVID-19 pandemic. However, the complexity of self-testing with nasopharyngeal diagnostics remains a challenge for certain individuals. Complicated instructions, discomfort associated with nasal or throat swabs, and visually interpreted tests create barriers for a substantial portion of the United States' 42.5 million disabled citizens when it comes to self-testing for respiratory viruses.
In response to this critical need, Magnasense has collaborated with the National Institute of Health's (NIH) Rapid Accessible Diagnostics (RADx) program. Together, we are pioneering an accessible saliva-based test for COVID, Flu A, and Flu B.
Magnasense's testing platform is designed to cater to individuals with visual, auditory, and motor coordination limitations, ensuring that elderly, young, or disabled users can effortlessly and swiftly self-test at a low cost.

Leveraging our expertise in therapeutic drug monitoring of biologic drugs and our prior research in saliva diagnostics, our dedicated team is expeditiously bringing this accessible test to market.
Magnasense is committed to breaking down barriers to respiratory virus testing, making it universally accessible and user-friendly for individuals of all abilities. Join us in advancing accessible diagnostics for a healthier, more inclusive future.
Health Check. Precision. Simplicity.
Our platform makes precision diagnostic testing and monitoring as easy as brushing your teeth
Unlocking a spectrum of possibilities, our innovative product platform enables the creation of integrated, user-friendly saliva-based tests for personalized diagnostics and monitoring.